Enantiocomplementary decarboxylative hydroxylation combining photocatalysis and whole-cell biocatalysis in a one-pot cascade process†
Abstract
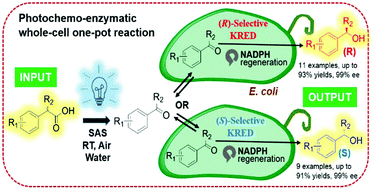
Designing a green, highly efficient and stereoselective catalytic system to generate valuable enantioenriched products is a long-standing goal in green chemistry. Here, we report a one-pot cascade combining photocatalysts with (R)- or (S)-selective ketoreductases for the decarboxylative carbonylation of carboxylic acids and the subsequent bioreduction to generate valuable chiral alcohols. Using this approach, various chiral alcohols with complementary (R)- or (S)-configurations were prepared with good yields (up to 93%) and excellent stereoselectivity (up to 99% ee). Such a photochemo-enzymatic one-pot whole-cell process combines the advantages of both photocatalysts and enzyme catalysts and provides a mild, green, metal-free and highly stereoselective alternative in asymmetric decarboxylative hydroxylation reactions.


 Please wait while we load your content...
Please wait while we load your content...