Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series of l-phenylalanine-derived surface-active ionic liquids †
Abstract
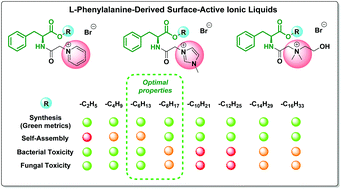
We report for the first time a comprehensive study on the synthesis (supported by green chemistry metrics), aggregation properties, bacterial/fungal toxicities and preliminary data on biodegradation of a series of 24 L-phenylalanine derived surface-active ionic liquids (SAILs). The various cationic headgroups included pyridinium, imidazolium, and cholinium groups and enabled a comprehensive analysis of the effect of the alkyl ester chain (from C2 to C16) on the synthesis, toxicity, biodegradability, and surfactant properties of the novel SAILs. The evaluation of the SAILs revealed that a wide variety of properties were strictly dependent on the side chain length, including their bacterial and fungal toxicities (from low toxicity to high toxicity), and aggregation properties. Addition of the L-phenylalanine moiety which connects the lipophilic side chain to the cationic head group results in the phenyl group essentially contributing to the self-assembling properties. The interplay of dispersion interactions of the phenyl ring and the side chain hydrophobicity allows us to rank the novel SAILs (thus identifying the remarkable ones) as compared to other surfactants. The CMC values for the SAILs reported in this study are significantly (up to 10 times) lower than those reported for conventional surfactants with the same length of the side chain. Adsorption and micellization are among the factors affecting the toxicity of the studied SAILs. Preliminary biodegradation studies have shown that no clear trend was observed when comparing the closed bottle test results of the SAIL C2 and C10 derivatives. Medium chain length (C6 to C8) pyridinium SAILs have been recommended as the most prospective green alternatives for conventional cationic surfactants. These findings can contribute to designing new efficient amphiphiles with optimized antimicrobial activities and to employ them as potential environmentally benign mineralisable surfactants.


 Please wait while we load your content...
Please wait while we load your content...