Solventless and metal-free regioselective hydrofluorination of functionalized alkynes and allenes: an efficient protocol for the synthesis of gem-difluorides†
Abstract
The combination of two easily handled, highly acidic liquid HF complex reagents, DMPU-12HF and KHSO4-13HF, generated a highly acidic fluorination system that facilitated exclusive Markovnikov addition of HF to widely functionalized alkynes, including alkyne tethered drugs, or allenes to produce gem-difluorides with high atom economy, and with an easy workup.
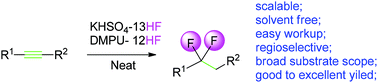


 Please wait while we load your content...
Please wait while we load your content...