Metal-free synthesis of triarylated (Z)-nitrones via H2O-mediated 1,3-dipolar transfer under aerobic conditions†
Abstract
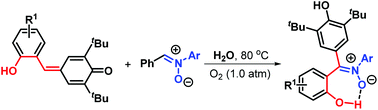
A new and environmentally benign protocol aimed at the generation of triarylated (Z)-nitrones in generally good yields has been developed via metal- and catalyst-free H2O-mediated 1,3-dipolar transfer reaction of para-quinone methides (p-QMs) with diarylated nitrones under aerobic conditions. The purification of these products only needs to be recrystallized by a mixed solvent comprising small amounts of petroleum ether and ethyl acetate, thereby avoiding the requirement of traditional chromatography. This new 1,3-dipolar strategy features broader substrate scope, green process, and mild conditions.


 Please wait while we load your content...
Please wait while we load your content...