Production of 5α-androstene-3,17-dione from phytosterols by co-expression of 5α-reductase and glucose-6-phosphate dehydrogenase in engineered Mycobacterium neoaurum†
Abstract
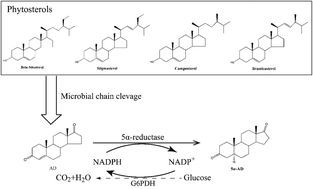
5α-Androstene-3,17-dione (5α-AD), an important intermediate in the synthesis of steroid drugs, is mainly synthesized by chemical methods in industry. In this work, a new biotechnological production method of 5α-AD from cheap precursors had been developed. 5α-Reductase from Treponema denticola, which can convert the 3-oxo-4-ene structure into 5α-reductase products, was screened and expressed in MNR M3Δksdd, a major AD-producing strain. The engineered MNR M3Δksdd/261-5αT. denticola strain showed a remarkably efficient one-step transformation of phytosterols (PS) into 5α-AD. As a cofactor for the reaction of 5α-reductase, nicotinamide adenine dinucleotide phosphate (NADPH) was supplied by the overexpression of glucose-6-phosphate dehydrogenase (G6PDH) in MNR M3Δksdd. Finally, the conversion increased from 67.9% to 86.4% in the cofactor recycling system. This study is the first to report the transformation of PS into 5α-AD by biological methods with a considerable yield. The present study also revealed that the engineered M. neoaurum containing 5α-reductase and G6PDH shows a practical value in producing 5α-AD in industry and provides a reference for the industrial production of high value products from cheap raw materials.


 Please wait while we load your content...
Please wait while we load your content...