Hetero-bifunctional catalyst manipulates carbonyl and alkynyl reductions of conjugated alkynones in an aqueous medium†
Abstract
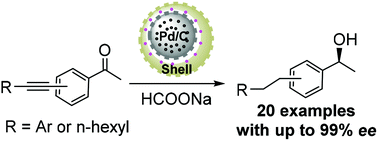
Selective manipulation of carbonyl and alkynyl reductions in conjugated alkynones and controllable multi-step organic transformations are particularly attractive in green catalysis. In this study, using yolk–shell-structured silica, we design a mesoporous silica-supported active site-isolated bifunctional catalyst with Pd/C species on the yolk encapsulated by chiral Ru(II)/diamine species in a silica shell. Structural analyses determine well-defined chiral single-site Ru(II)/diamine species anchored in the nanochannels of silica shells, whereas electron microscopy characterizations disclose the uniformly distributed yolk–shell-structured morphology. As a dual reductive catalyst, it enables selective and successive carbonyl and alkynyl reductions of conjugated alkynones via a controllable catalysis sequence. The cascade reaction initially goes through Ru-catalyzed asymmetric transfer hydrogenation followed by a Pd/C-catalyzed reduction process to afford various chiral aromatic alcohols in high yields with up to 99% enantioselectivity in an aqueous medium. Furthermore, the designed functionalities of the catalyst including ethylene-bridged hydrophobicity, uniformly dispersed morphology, and dual acitve centers make integrated contribution to its catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...