Base-promoted ring-closing carbonyl–allene metathesis for the synthesis of 2,4-disubstituted pyrroles†
Abstract
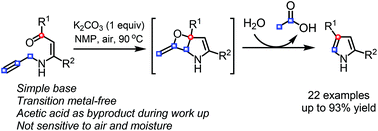
A base-promoted ring-closing carbonyl–allene metathesis reaction of N-allenyl β-enaminones (generated in situ from N-propargyl β-enaminones) gives 2,4-disubstituted pyrroles with cleavage of the C(sp)–C(sp3) bond. This transition metal-free procedure generates 1 equiv. of acetic acid as the sole byproduct. A preliminary mechanistic study supports a stepwise [2 + 2] cycloaddition/retro [2 + 2] reaction pathway.


 Please wait while we load your content...
Please wait while we load your content...