A methodical selection process for the development of ketones and esters as bio-based replacements for traditional hydrocarbon solvents†
Abstract
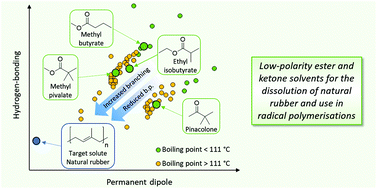
A “top down” approach to the development of sustainable, greener, low-polarity solvents is presented. Methyl butyrate, ethyl isobutyrate, methyl pivalate and pinacolone were identified as potential target solvents from trends in Hansen solubility parameters and known physical properties. Solubility, flammability and physical properties were determined which showed their potential to replace traditional, hazardous, volatile, non-polar solvents such as toluene. Each new candidate then demonstrated their suitability to replace these traditional solvents in solubility tests, despite being esters and ketones, each candidate demonstrated their similarity to traditional volatile non-polar solvents in terms of their solubility properties by their ability to dissolve natural rubber, a particularly low-polarity solute. This was reinforced by their performance in a model Menschutkin reaction and a radical-initiated polymerisation for the production of pressure-sensitive adhesives, where their performance was found to be similar to that of toluene. Importantly, a preliminary toxicity test (Ames test) suggested non-mutagenicity in all candidates. Each of the four candidates can be synthesised via a catalytic route from potentially renewable resources, thus enhancing their green credentials.


 Please wait while we load your content...
Please wait while we load your content...