Reply to the ‘Comment on “Zemplén transesterification: a name reaction that has misled us for 90 years”’ by G. Poli, C. Pezzetta, I. Leito and S. Tshepelevitsh, Green Chemistry, 2018, 20, DOI: 10.1039/c7gc03795c †
Abstract
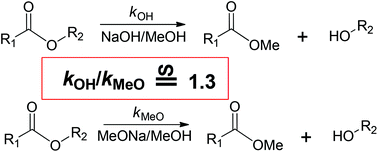
Prof. Poli and co-workers recently commented on our previous report in which we demonstrated that using NaOH and NaOMe in methanol for deacylation are identical. By consideration of the equilibrium between methoxyl and hydroxyl anions, they proposed that the methoxyl anion-catalysed mechanistic path still holds in the deacylation when using NaOH. In theory we believe that their explanation is reasonable. However, our experimental results showed higher transesterification rates when using NaOH than when using MeONa, which may indicate that the hydroxyl anion-catalysed mechanistic path also plays a key role when using NaOH.


 Please wait while we load your content...
Please wait while we load your content...