p-TsOH promoted synthesis of benzo-fused O-heterocycles from alkynols via ring contraction and C–O scission strategy†
Abstract
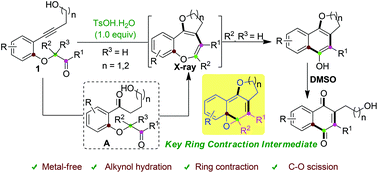
Here, we report the first divergent synthesis of benzo-fused O-heterocycles by p-toluene sulfonic acid promoted cascade reactions involving alkyne hydration, double cyclization, ring contraction and C–O bond cleavage from alkynols. The reaction mechanism was validated by obtaining the X-ray structure of fused benzo[b]oxepine intermediates. Moreover, some of the obtained derivatives could be transformed into 2,3-disubstituted naphthoquinones as important structural motifs in natural products and bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...