Catalyst-free nucleophilic addition reactions of silyl glyoxylates in water†
Abstract
A novel catalyst-free addition of thiols to silyl glyoxylates was developed in water, providing an efficient route for the synthesis of α-hydroxysilanes. In the activation of silyl glyoxylates, hydrogen bonding is critical to the reaction, and rate acceleration was observed in water. Moreover, the competing Brook rearrangement was significantly suppressed with the assistance of water.
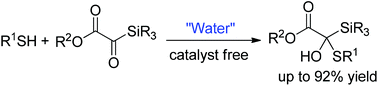


 Please wait while we load your content...
Please wait while we load your content...