Facile synthesis of 1,4-diketones via three-component reactions of α-ketoaldehyde, 1,3-dicarbonyl compound, and a nucleophile in water†
Abstract
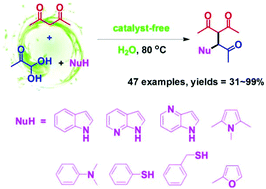
Three-component reactions of alkylglyoxals, 1,3-dicarbonyl compounds, and a nucleophile were performed under aqueous and catalyst-free conditions, which produced 1,4-diketone scaffolds in a straightforward way. Many compounds, such as indole, azaindole, pyrrole, 2-methylfuran, N,N-dimethylaniline, N-methylaniline, thiophenol, and benzyl mercaptan, were all able to act as nucleophiles to react with alkylglyoxal and 1,3-dicarbonyl compounds. The mechanim of this reaction was also investigated. The obtained 1,4-diketones can be easily converted to many valuable chemicals.


 Please wait while we load your content...
Please wait while we load your content...