Lignin extraction and catalytic upgrading from genetically modified poplar†
Abstract
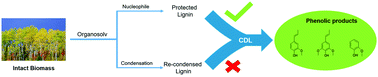
Lignin as the only natural resource of aromatics has the potential to be the feedstock of choice for the production of value-added chemicals. However, the recalcitrance of lignin has limited its valorization into value-added products, and it is often burned for heat. The acid-catalyzed organosolv extraction of lignin results in the formation of interunit carbon–carbon bonds, which limit its upgrading. In this work, three different solvent systems (methanol, acetone, and acetic acid) were evaluated for the extraction of native lignin from wild-type and genetically modified poplar species (wild type, high-S, and low-S). Over 68% of the original lignin in the biomass was isolated and subjected to further upgrading over a heterogeneous Ni/C catalyst (10 wt% of catalyst in methanol solvent, under 35 bar H2 pressure at 225 °C). Three major monomeric phenolic products, guaiacol, isoeugenol, and 4-propenyl syringol, were obtained. Methanol-extracted lignin gave the best yield of >60% of the said aromatic products. Methanol as a nucleophile reacted with the Cα benzylic carbocation formed during the organosolv extraction, minimizing the carbon–carbon bond formation. This protection by methanol was demonstrated by NMR spectroscopy. Scanning electron microscopy (SEM) images showed differences in the isolated lignin on the micron scale from the three different treatments.


 Please wait while we load your content...
Please wait while we load your content...