An efficient palladium catalyzed Mizoroki–Heck cross-coupling in water†
Abstract
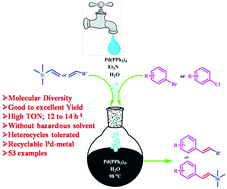
The homogeneous Pd-catalysed Mizoroki–Heck coupling reaction was successfully performed in water in the absence of any additives under aerobic conditions. The various key reaction parameters that affect the yield of the desired cross-coupling product were optimized. The Pd(PPh3)4/Et3N/H2O/98 °C catalyst system was found to be highly active (TOF = 12 to 14 h−1) towards achieving excellent yield of the Mizoroki–Heck coupling products for a wide range of electron-withdrawing as well as electron-donating aryl bromides and chlorides in the shortest reaction time. Pd(PPh3)4 catalyst deactivation during the Mizoroki–Heck coupling reaction was investigated and to evolve a strategy for achieving ten times Pd-metal recyclability without appreciable loss of its activity. Thus, the proposed mechanism provides access to a variety of olefins in aqueous medium, making this protocol eco-friendly.


 Please wait while we load your content...
Please wait while we load your content...