Unusual differences in the reactivity of glutamic and aspartic acid in oxidative decarboxylation reactions†
Abstract
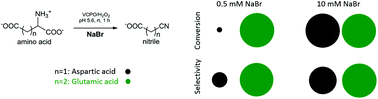
Amino acids are potential substrates to replace fossil feedstocks for the synthesis of nitriles via oxidative decarboxylation using vanadium chloroperoxidase (VCPO), H2O2 and bromide. Here the conversion of glutamic acid (Glu) and aspartic acid (Asp) was investigated. It was observed that these two chemically similar amino acids have strikingly different reactivity. In the presence of catalytic amounts of NaBr (0.1 equiv.), Glu was converted with high selectivity to 3-cyanopropanoic acid. In contrast, under the same reaction conditions Asp showed low conversion and selectivity towards the nitrile, 2-cyanoacetic acid (AspCN). It was shown that only by increasing the amount of NaBr present in the reaction mixture (from 0.1 to 2 equiv.), could the conversion of Asp be increased from 15% to 100% and its selectivity towards AspCN from 45% to 80%. This contradicts the theoretical hypothesis that bromide is recycled during the reaction. NaBr concentration was found to have a major influence on reactivity, independent of ionic strength of the solution. NaBr is involved not only in the formation of the reactive Br+ species by VCPO, but also results in the formation of potential intermediates which influences reactivity. It was concluded that the difference in reactivity between Asp and Glu must be due to subtle differences in inter- and intramolecular interactions between the functionalities of the amino acids.


 Please wait while we load your content...
Please wait while we load your content...