A regioselective one-pot aza-Friedel–Crafts reaction for primary, secondary and tertiary anilines using a heterogeneous catalyst†
Abstract
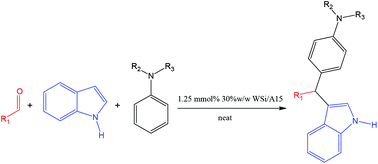
A one-pot multicomponent aza-Friedel–Crafts reaction was performed under green, neat and heterogeneous conditions in the presence of 1.25 mmol% of 30% w/w silicotungstic acid supported on Amberlyst 15 beads. In a yet unprecedented attempt, both primary and secondary anilines could provide the 3-substituted indole product in good to excellent yields usually at room temperature whilst tertiary anilines gave moderate results. Interestingly all reactions proceeded regioselectively via two separate C–C bond forming reactions rather than C–C and C–N bond forming steps. The catalyst is easy, safe and environmentally-benign to prepare, completely recoverable and recyclable up to 5 times.


 Please wait while we load your content...
Please wait while we load your content...