Visible light-induced C–H sulfenylation using sulfinic acids†
Abstract
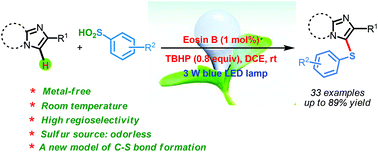
The visible light and Eosin B-catalyzed direct sulfenylation of sp2 C–H bonds with sulfinic acids through a photoredox process has been demonstrated at room temperature under transition metal-free conditions for the first time. Diverse heteroaryl sulfides were obtained in moderate to good yields. This is the first example of the sulfenylation of sp2 C–H bonds using arylsulfinic acids as odorless sulfur reagents under visible light-induced conditions. More interestingly, the reductive products could be obtained under visible light-induced oxidative conditions. This protocol demonstrates a new model for C–S bond formation, which serves as a novel approach toward the synthesis of heteroaryl sulfides.


 Please wait while we load your content...
Please wait while we load your content...