Synthesis of isoquinolones via Rh-catalyzed C–H activation of substituted benzamides using air as the sole oxidant in water†
Abstract
Most of the metal-catalyzed C–H activation/annulation reactions were carried out in organic solvents using expensive oxidants such as Cu(II) and Ag(I) salts. Here, we reported a new approach for a highly regioselective synthesis of isoquinolones from N-alkyl benzamides and alkynes using an Rh(III) catalyst and inexpensive oxygen as the sole oxidant in an aqueous medium. In the reaction, water gave the highest product yield among the solvents used. In addition, at the end of the reaction, the isoquinolone product directly precipitated out from the aqueous solution. The methodology can be applied to a gram scale synthesis. This Rh(III)-catalyzed reaction shows interesting meta selectivity with the meta substituted benzamide and shows various regioselectivities with different substituted alkynes. Moreover, the methodology can be applied to the preparation of biologically active compounds having the isoquinolone core.
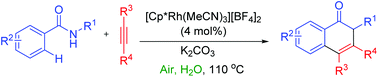


 Please wait while we load your content...
Please wait while we load your content...