A Schiff base-modified silver catalyst for efficient fixation of CO2 as carboxylic acid at ambient pressure†
Abstract
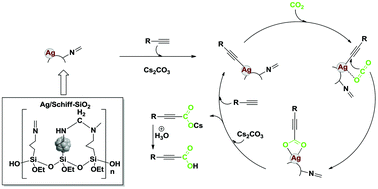
The fixation of CO2 as a carboxylic acid is a significant reaction for C–C bond formation in organic synthesis. So far, besides C–H carboxylations using stoichiometric amounts of metals or organometallic reagents, great efforts have been devoted to develop new heterogeneous catalyst, while the catalytic performance of supported metal catalysts is not satisfactory. Herein, a Schiff base-modified silver catalyst was developed for the direct carboxylation of terminal alkynes with CO2, enabling an efficient and green synthesis of valuable alkynyl carboxylic acids. The reaction can smoothly proceed under atmospheric pressure and low temperature (60 °C) conditions. Moreover, a silver-based catalyst, which was prepared by an in situ reduction route, can be easily prepared, recovered, and reused five times without significant loss of activity due to the stability promoted by the Schiff-base on the support surface. In addition, the markedly negative influence of H2O and solvent effect on this reaction system has also been discussed.

 Please wait while we load your content...
Please wait while we load your content...