Mechanochemical indole synthesis by rhodium-catalysed oxidative coupling of acetanilides and alkynes under solventless conditions in a ball mill†
Abstract
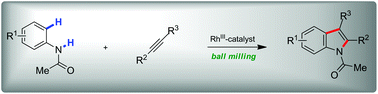
A mechanochemical indole synthesis by rhodium(III)-catalysed C–H bond functionalisation in a planetary mill has been developed. It occurs in the absence of any solvent, does not require additional heating and only needs catalytic quantities of Cu(OAc)2 in combination with dioxygen as a terminal oxidant. Accordingly, the process represents a powerful and environmentally benign alternative to the common solution-based standard protocols.


 Please wait while we load your content...
Please wait while we load your content...