Towards environmentally acceptable synthesis of chiral α-hydroxy ketones via oxidase-lyase cascades†
Abstract
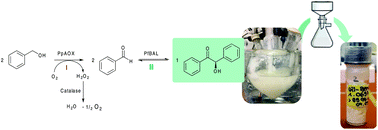
The one-pot multistep enzymatic oxidation of aliphatic and benzylic alcohols to the corresponding aldehydes combined with their subsequent carboligation to chiral α-hydroxy ketones has been exemplarily evaluated in terms of being a “green” biocatalytic approach. Besides the potential to start from bio-derived alcohols, this concept avoids the direct use of the reactive aldehyde intermediates, enables addition of high substrate concentrations in one liquid phase while maintaining enzyme activity and enables a simplified product isolation with diminished waste formation.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...