Functionality and molecular weight distribution of red oak lignin before and after pyrolysis and hydrogenation†
Abstract
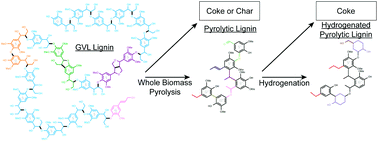
Three red oak derived lignin samples: 1. lignin extracted from red oak chips using γ-valerolactone (GVL lignin), 2. lignin extracted from the pyrolysis oil of red oak chips by fractionation and water extraction (pyrolytic lignin) and 3. pyrolytic lignin hydrogenated over Ru/C (hydrogenated pyrolytic lignin), were analyzed by FT-ICR MS, NMR, and GPC. More than 1100 distinct molecular weights were observed by FT-ICR MS of the lignin streams while changes in the O/C and H/C ratios suggested the dehydration of hydroxylated sidechains from pyrolysis and partial saturation of the compounds from hydrogenation. The relative average molecular weight of the lignin determined by GPC decreased five-fold after pyrolysis. Quantitative 13C, HSQC, and HMBC NMR revealed a decrease in the C–O aliphatics from pyrolysis potentially forming alkane, alkene, and carbonyl functionalities. The aldehydes and ketones were highly reactive during hydrogenation and may potentially be responsible for coke formation.


 Please wait while we load your content...
Please wait while we load your content...