Metal-free selenosulfonylation of alkynes: rapid access to β-(seleno)vinyl sulfones via a cationic-species-induced pathway†
Abstract
A novel and convenient method has been developed for the 1,2-selenosulfonylation of alkynes under metal-free conditions. The reaction avoids the need for the pre-prepared Se–S reagents, providing facile access to a series of β-(seleno)vinyl sulfones with high levels of regio- and stereoselectivity. The key features of this reaction include broad substrate scope, excellent functional-group tolerance, readily available reagents and amenability to gram-scale synthesis. Preliminary mechanistic studies support the proposed cationic-species-induced pathway.
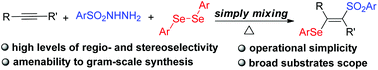


 Please wait while we load your content...
Please wait while we load your content...