A nanoporous nickel catalyst for selective hydrogenation of carbonates into formic acid in water†
Abstract
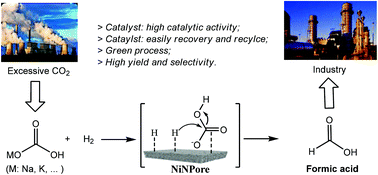
An efficient unsupported nanoporous nickel (NiNPore) material for the hydrogenation of carbonates to formic acid (FA) in water was investigated for the first time. NiNPore is an environmentally benign catalyst and it exhibited remarkable catalytic activity in the reduction of a wide range of carbonates to afford formic acid in excellent yields with high selectivity, and maximum values of 86.6% from NaHCO3 and even up to 92.1% from KHCO3 were obtained. The hydrogen pressure and pKa of the carbonates had a significant influence on the formation of FA. The catalyst was easily recovered and could be recycled at least five times without leaching and loss of activity. The present study demonstrated a potential application for the synthesis of FA from CO2 or carbonate compounds.

 Please wait while we load your content...
Please wait while we load your content...