Expanding the reaction space of aldolases using hydroxypyruvate as a nucleophilic substrate†
Abstract
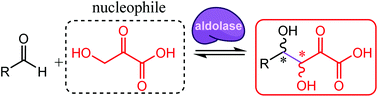
Aldolases are key biocatalysts for stereoselective C–C bond formation allowing access to polyoxygenated chiral units through direct, efficient, and sustainable synthetic processes. The aldol reaction involving unprotected hydroxypyruvate and an aldehyde offers access to valuable polyhydroxy-α-keto acids. However, this undescribed aldolisation is highly challenging, especially regarding stereoselectivity. This reaction was explored using, as biocatalysts, a collection of aldolases selected from biodiversity. Several enzymes that belong to the same pyruvate aldolase Pfam family (PF03328) were found to produce the desired hexulosonic acids from hydroxypyruvate and D-glyceraldehyde with complementary stereoselectivities. One of them was selected for the proof of concept as a biocatalytic tool to prepare five (3S,4S) aldol adducts through an eco-friendly process.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...