Aldehyde/ketone-catalyzed highly selective synthesis of 9-monoalkylated fluorenes by dehydrative C-alkylation with primary and secondary alcohols†
Abstract
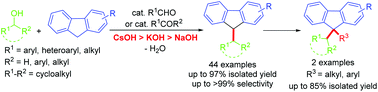
By using aldehydes or ketones as the catalyst and screening CsOH out as the more effective base than KOH in many instances, an efficient 9-C-alkylation of fluorenes with alcohols was achieved to provide a green and practical method for general synthesis of the useful 9-monoalkylated fluorenes in high selectivities. This new method tolerates a wide range of substrates including activated and unactivated primary and secondary alcohols, thus solving the issues remaining in the field and largely broadening the diversity of the 9-monoalkylated fluorenes. Consequently, fine-tuning of the alkylated fluorenes was made possible to provide specific fluorene monomers for function-oriented polyfluorenes. Preliminary mechanistic studies revealed that the external carbonyl compounds can be quantitatively regenerated and recovered in the reaction cycle.

 Please wait while we load your content...
Please wait while we load your content...