A catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles: an easy access to five-ring-fused tetrahydroisoquinolines†
Abstract
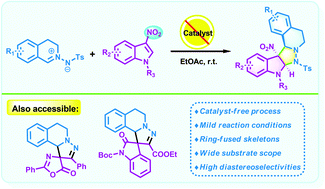
We have reported herein a catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles by which a series of five-ring-fused tetrahydroisoquinolines featuring an indoline scaffold were obtained as single diastereomers in moderate to high yields without any additives under mild conditions. Moreover, the current method provides a novel and convenient approach for the efficient incorporation of two biologically important scaffolds (tetrahydroisoquinoline and indoline).
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles

 Please wait while we load your content...
Please wait while we load your content...