Quantitative chemocatalytic production of lactic acid from glucose under anaerobic conditions at room temperature†
Abstract
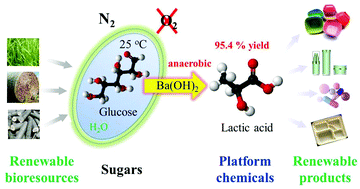
Chemical conversion of glucose into lactic acid in water requires harsh reaction conditions to obtain relatively low product yields. Herein, a simple, but highly efficient chemocatalytic process is reported for the production of lactic acid from glucose in the presence of Ba(OH)2 under a nitrogen atmosphere at 1 bar total pressure, where glucose was selectively converted to lactic acid with a yield of 95.4% at room temperature in 48 h. The process was applied to cellobiose, fructose, dihydroxyacetone, glyceraldehyde, pyruvaldehyde and cellulose hydrolysate, among which pyruvaldehyde afforded ca. 100% lactic acid yield. Product distribution changed towards glyceric acid, glycolic acid, formic acid, malonic acid and CO2, by variation of the O2 partial pressure, which promotes the oxidation of glyceraldehyde and 1,3-dihydroxyacetone intermediates. The process developed has significant advantages over previous methods in aspects of efficiency, conditions, reactor materials and productivity.

 Please wait while we load your content...
Please wait while we load your content...