Self-curing furan-based elastic thermosets derived from citric acid†
Abstract
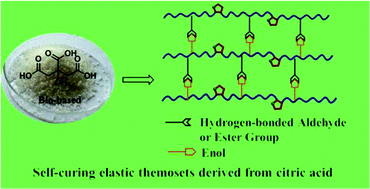
A series of self-curing furan-based polyesters were prepared from naturally occurring citric acid. Citric acid was firstly converted to dimethyl 1,3-acetonedicarboxylate through a one pot, two-step process in high yield, which was subsequently used for the synthesis of methyl 3-(methoxycarbonyl)furan-2-acetate (MCFA) via Feist–Benary synthesis. By reacting MCFA with bio-based α,ω-diols HO-(CH2)m-OH (m = 2, 3, 4, 10) via a transesterification method, thermoplastic polyesters (named PE-ms) with no crystallinity, high viscosity and strong thermal resistance were obtained. Surprisingly, the obtained polyesters underwent self-curing reactions without adding any catalyst or applying an external stimulus, leading to rubberlike thermoset polymers. Chemical transformation of MCFA and PE-ms before and after self-curing reactions was investigated to demonstrate the curing mechanism using FT-IR, 1H NMR spectroscopy, etc. Results indicated that the curing reactions were caused by the hydrogen bond interactions between keto–enol tautomerizing of the β-ketoglutarate structures generated by ring opening reactions of furan rings along the polymer chains. In addition, the thermal properties, mechanical behavior as well as the biodegradability of the obtained thermosets were evaluated by thermogravimetric analyses (TGA), differential scanning calorimetry (DSC), dynamic mechanical analyses (DMA), tensile tests and biodegradation experiments. These self-curing thermoset polyesters are potentially useful as one-component coating, elastomers, and adhesives.

 Please wait while we load your content...
Please wait while we load your content...