Suitability of bio-based ionic liquids for the extraction and purification of IgG antibodies†
Abstract
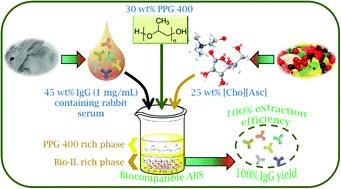
In the past decade, remarkable advances in the production and use of antibodies as therapeutic drugs and in research/diagnostic fields have led to their recognition as value-added proteins. These biopharmaceuticals have become increasingly important, reinforcing the current demand for the development of more benign, scalable and cost-effective techniques for their purification. Typical polymer–polymer and polymer–salt aqueous biphasic systems (ABS) have been studied for such a goal; yet, the limited polarity range of the coexisting phases and their low selective nature still are their major drawbacks. To overcome this limitation, in this work, ABS formed by bio-based ionic liquids (ILs) and biocompatible polymers were investigated. Bio-based ILs composed of ions derived from natural sources, namely composed of the cholinium cation and anions derived from plants natural acids, have been designed, synthesized, characterized and used for the creation of ABS with polypropyleneglycol (PPG 400). The respective ternary phase diagrams were initially determined at 25 °C to infer on mixture compositions required to form aqueous systems of two phases, further applied in the extraction of pure immunoglobulin G (IgG) to identify the most promising bio-based ILs, and finally employed in the purification of IgG from complex and real matrices of rabbit serum. Remarkably, the complete extraction of IgG to the IL-rich phase was achieved in a single-step. With pure IgG a recovery yield of 100% was obtained, while with rabbit serum this value slightly decreased to ca. 85%. Nevertheless, a 58% enhancement in the IgG purity was achieved when compared with its purity in serum samples. The stability of IgG before and after extraction was also evaluated by size exclusion high-performance liquid chromatography (SE-HPLC), sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and Fourier transform infrared spectroscopy (FTIR). In most ABS formed by bio-based ILs, IgG retained its native structure, without degradation or denaturation effects, supporting thus their potential as remarkable platforms for the purification of high-cost biopharmaceuticals.


 Please wait while we load your content...
Please wait while we load your content...