Arbutin-based benzoxazine: en route to an intrinsic water soluble biobased resin†
Abstract
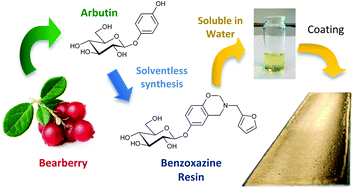
A novel benzoxazine monomer containing a carbohydrate moiety was synthesized using a solventless approach from the reaction of arbutin, a naturally occurring phenolic compound, furfurylamine and formaldehyde. The chemical structure of this fully bio-based benzoxazine monomer was confirmed by 1H NMR and FTIR. Its polymerization has been investigated and monitored by DSC showing the ring opening polymerization of benzoxazine functions with a network densification thanks to the reaction of the furan group. The high hydroxyl content of the monomer allows its easy solubilisation in water paving the way to a wide range of possible “environmentally friendly” applications especially in the fields of coatings, paintings and adhesives.

 Please wait while we load your content...
Please wait while we load your content...