CO from CO2 and fluctuating renewable energy via formic-acid derivatives†
Abstract
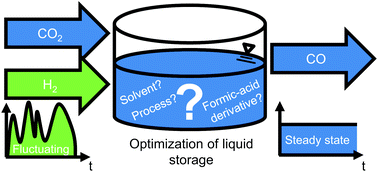
Integrating fluctuating renewable energy into continuously operating industries requires energy storage. Energy storage can be achieved by using hydrogen from fluctuating, renewable energy for hydrogenation of CO2. The resulting molecule serves as storage. The simplest molecule that can be stored in liquid form is formic acid. Stored formic acid can then be reformed continuously to carbon monoxide, a common feedstock for the chemical industry. Since formic-acid synthesis is thermodynamically challenging, we investigate alternative storage molecules such as formamides or formates. Currently, it is unknown which storage molecule leads to the most efficient storage process. Thus, we systematically identify the most efficient storage molecule, together with an optimal combination of solvent and process flowsheet. We identify this combination with a novel hierarchical model-based approach, which starts by screening with the predictive thermodynamic model COSMO-RS and ends by using experimental property data. In the novel approach, we evaluate more than 100 000 combinations of storage molecules, solvents and process flowsheets. The most efficient combination identified uses the storage molecule N,N-dimethylformamide, and reduces the exergy loss by more than a factor of 15 compared to storage of formic acid, and still 65% compared to a literature benchmark. The largely reduced exergy loss indicates an environmentally promising route for linking fluctuating, renewable energy with continuously operating chemical industries. Our findings therefore highlight the importance of catalyst development for N,N-dimethylformamide in the optimal solvents.

 Please wait while we load your content...
Please wait while we load your content...