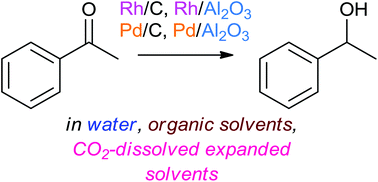
Selective hydrogenation of acetophenone with supported Pd and Rh catalysts in water, organic solvents, and CO2-dissolved expanded liquids†
Abstract
Solvent effects were investigated for selective hydrogenation of acetophenone (AP) with commercial 5% Pd/Al2O3 and 5% Pd/C catalysts using polar and nonpolar solvents. The rate of AP hydrogenation varied with the solvents used in different ways depending on the catalysts used. The highest AP conversion was achieved with water for the two catalysts and the AP conversion was correlated with hydrogen-bond-acceptance (HBA) capability (β) for Pd/Al2O3 and hydrogen-bond-donation (HBD) capability (α) for Pd/C. These trends were the same as the corresponding solvent effects obtained previously with Rh/Al2O3 and Rh/C catalysts, respectively (Green Chem., 2015, 17, 1877–1883). The influence of solvents on the rate of AP hydrogenation depends on the support materials and not on the metal species. Furthermore, AP hydrogenation was conducted with the four supported noble metal catalysts in different solvents pressurized by CO2 (CO2-dissolved expanded solvents). The influence of CO2 pressurization on the rate of reaction, the product selectivity, and the catalyst life was investigated to know whether or not CO2 molecules could function as a reaction promoter in heterogeneous AP hydrogenation reactions with supported Pd and Rh catalysts in different solvents.

 Please wait while we load your content...
Please wait while we load your content...