Transition metal-free, iodide-mediated domino carbonylation–benzylation of benzyl chlorides with arylboronic acids under ambient pressure of carbon monoxide†
Abstract
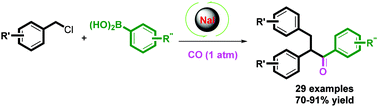
A conceptually distinct approach toward the synthesis of 1,2,3-triarylpropan-1-one by NaI-catalyzed domino carbonylation–benzylation of unactivated benzyl chlorides with arylboronic acids has been developed under ambient pressure of CO gas. The novel method represents a significant improvement over the traditional palladium-catalyzed carbonylation: the catalyst NaI is abundant, inexpensive, and bench stable, the reaction conditions are much milder, and the use of ligands and the costly need to remove metallic impurities from the end products are avoided. This apparently transition-metal-free process offers a new strategy for carbonylative cross-coupling reactions of C(sp3) halides.

 Please wait while we load your content...
Please wait while we load your content...