Synthesis of N-alkyl-4-vinylpyridinium-based cross-linked polymers and their catalytic performance for the conversion of fructose into 5-hydroxymethylfurfural†
Abstract
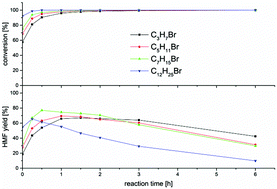
We developed a new acid-free and metal-free heterogeneous catalytic system for the dehydration of fructose into 5-hydroxymethylfurfural (HMF). These catalysts are ionic polymers based on 4-vinylpyridine, crosslinked with divinylbenzene. The effect of different polymer properties, like the alkyl chain length at the pyridinium nitrogen, the type of anion, the porosity, the degree of post polymerization modification as well as the effect of temperature was investigated. Poly(N-alkylvinylpyridinium bromides) showed the best results. A maximum HMF yield of 77% after 0.5 h at 180 °C, using ethanol as the low boiling solvent, was achieved. Most of the byproducts are the acetals of HMF with the alcohol medium, which are often converted in downstream processes in the same manner as HMF. Thus, one can effectively consider the catalyst system as almost fully selective to HMF.

 Please wait while we load your content...
Please wait while we load your content...