Methyl NFSI: atom-economical alternative to NFSI shows higher fluorination reactivity under Lewis acid-catalysis and non-catalysis†
Abstract
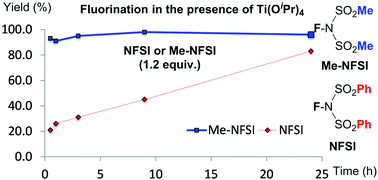
Me-NFSI was first reported in 1994. Despite its atom-economical structure and similarity to a well-explored fluorinating reagent, NFSI, Me-NFSI has not appeared in the literature in over 20 years. We disclose that Me-NFSI is more effective for the fluorination of active methines under Lewis acid-catalysis and non-catalysis than NFSI.

 Please wait while we load your content...
Please wait while we load your content...