An efficient and recyclable thiourea-supported copper(i) chloride catalyst for azide–alkyne cycloaddition reactions†
Abstract
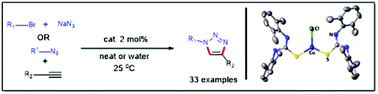
A reaction between two equivalents of bulky thiourea, i.e., 1,3-bis(2,6-dimethylphenyl)thiourea (L) [L = (ArNH)2C = S; Ar = 2,6-Me2C6H3], and CuCl2 or CuCl in THF solvent afforded a bulky thiourea-stabilized copper(I) halide complex, LCu(Cl)L (1). Compound 1 was characterized by NMR spectroscopy, mass spectrometry and X-ray structural analysis. Further, we tested the catalytic activity of this well-defined compound 1 for azide–alkyne cycloaddition (AAC) reactions in solvent-free conditions. Compound 1 shows a high catalytic activity for the synthesis of 1,4-di- and 1,4,5-trisubstituted 1,2,3-triazoles in good to excellent yields from azides (aliphatic or aromatic) and alkynes (both terminal and disubstituted). Furthermore, three-component CuAAC reactions were successfully carried out to obtain both 1,4-di- and 1,4,5-trisubstituted 1,2,3-triazoles in good to excellent yields from an organic halide, sodium azide and alkyne (both terminal and disubstituted) in greener solvent water. More importantly, the catalyst can be reused efficiently up to ten consecutive cycles with negligible loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...