Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes†
Abstract
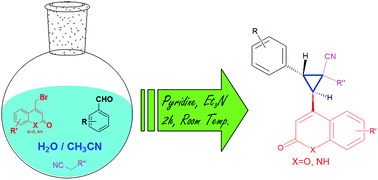
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles. The room temperature reaction in aqueous medium has been found to be diastereoselective and high yielding.

 Please wait while we load your content...
Please wait while we load your content...