Ionic liquids as solvents for PPTA oligomers†
Abstract
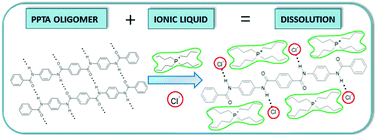
Poly-p-phenyleneterephthalamide (PPTA) is an aramid polymer with high tensile strength which is currently industrially synthesized in a solvent mixture of N-methylpyrrolidone (NMP) and CaCl2. Due to the toxicity of NMP and the need for a salt to increase the solubility, ionic liquids are suggested as suitable, alternative solvents. A whole series of ionic liquids (ILs) were investigated for their solubilization strength towards PPTA. For this study, small PPTA oligomers were synthesized and used as model compounds in solubility tests with ionic liquids. This study gave insights in the types of cations and anions required for optimal dissolving behavior. Ionic liquids with coordinating anions are a requirement to solubilize PPTA by disrupting the intermolecular hydrogen bond network, just as is the case for cellulose dissolution. Infrared and NMR-spectroscopic studies revealed the interaction of the anions with the hydrogen atoms of the secondary amides of the aramid chains. However, there is no one-to-one relationship between ionic liquids suitable for PPTA and cellulose dissolution. Cations with hydrogen atoms capable of hydrogen bond formation, like imidazolium cations, are poor solvents for PPTA. These cations hamper the anions in using their full potential for coordination with the oligomers. Ammonium and phosphonium ionic liquids which contain only sp3-bonded hydrogen atoms on the cation, do not show a tendency to form hydrogen bonds and dissolve PPTA oligomers much better than their imidazolium analogues. This hypothesis was further confirmed by the fact that substitution of hydrogen atoms by methyl groups on imidazolium and pyridinium cations improves the solvent power of the ionic liquid significantly. This screening test has identified several types of ionic liquids that are able to dissolve larger amounts of the PPTA oligomers on a molar basis than the currently used industrial solvent NMP/CaCl2.

 Please wait while we load your content...
Please wait while we load your content...