Removal and safe reuse of highly toxic allyl alcohol using a highly selective photo-sensitive metal–organic framework†
Abstract
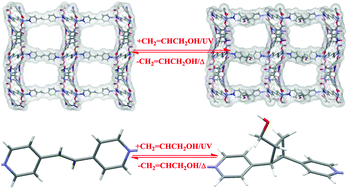
Herein, we report a facile and green approach to remove, convert and even release highly toxic allyl alcohol inside a MOF. The MOF used here, namely ECIT-20, barely adsorbs allyl alcohol under ambient conditions, whereas a sharp increase up to 90 mg g−1 (1.03 mol mol−1) under UV (297 nm) irradiation is observed, giving an ultra-big photo-switching behavior of more than 19 times. Thermogravimetric analysis, infrared spectroscopy, and 1H MAS plus 13C CPMAS solid-state NMR spectroscopy showed that a host–guest photochemical [2 + 2] reaction followed by the formation of asymmetric cyclobutanes is responsible for this unique photo-switching behavior towards allyl alcohol. The final conversion capability by means of ECIT-20 from allyl alcohol to asymmetric cyclobutanes is estimated to be 0.5 mol mol−1, which agrees well with both the structural features and the results from density functional theory (DFT) calculations. Moreover, the reuse of this MOF material is also facile and even after four cycles, excellent photo-switching performance towards allyl alcohol could be well maintained. This indicates that the highly promising material of ECIT-20 is suitable for the green removal and safe reuse of highly toxic allyl alcohol.

 Please wait while we load your content...
Please wait while we load your content...