Preparation of amphiphilic sorbitan monoethers through hydrogenolysis of sorbitan acetals and evaluation as bio-based surfactants†
Abstract
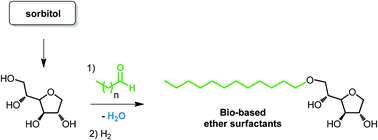
Amphiphilic sorbitan acetals have been prepared from sorbitan by acetalisation using linear aliphatic aldehydes or by transacetalisation starting from the corresponding aldehyde diethylacetals. A series of sorbitan acetals has been obtained with 29–81% isolated yields. It has been shown that these sorbitan acetals exist as a mixture of five-membered and six-membered regioisomers. Hydrogenolysis of the mixtures gave the corresponding sorbitan ethers as a mixture of 3 regioisomers with 55–85% isolated yields. A one-pot two-step procedure was also optimized from sorbitan giving similar results. The amphiphilic properties of sorbitan acetals and ethers were evaluated and both families exhibit interesting surfactant properties.

 Please wait while we load your content...
Please wait while we load your content...