Direct upgrading of fast pyrolysis lignin vapor over the HZSM-5 catalyst†
Abstract
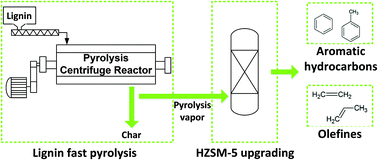
Lignin has been pyrolyzed in a continuous fast pyrolysis reactor and the vapor was subsequently upgraded in situ over a downstream, close coupled HZSM-5 catalyst in a fixed bed reactor. The effect of the catalyst temperature on the HZSM-5 upgrading of lignin derived pyrolysis vapor was investigated. The results show that a high catalyst temperature (600 °C) is required in order to produce oxygen free aromatics. At a catalyst temperature of 600 °C, an organic liquid product, which contains 70 wt% oxygen free aromatics (mainly benzene and toluene), is obtained. However, the yield of the organic liquid is reduced from 27.6 wt%daf without a catalyst to 5.7 wt%daf (600 °C catalyst temperature). The energy recovery in the liquid organics is 8.7% (600 °C catalyst temperature), compared to the 33.0% energy recovery in the organic liquid from the non-catalytic run. Oxygen is removed from the pyrolysis vapor mainly in the form of H2O and CO when using the HZSM-5 zeolite, which is less optimal compared to if CO2 was the product. The organic liquid fraction, obtained from the 600 °C catalyst temperature experiment, has a low oxygen content of 4.0 wt%, compared to the 23.4 wt% oxygen content in the untreated organic liquid.

 Please wait while we load your content...
Please wait while we load your content...