Organobase catalysis using 1-(2-pyrimidyl)piperazine in micellar medium: an approach for better performance and reusability of organobase†
Abstract
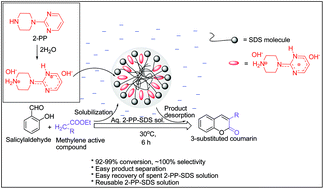
An organobase–surfactant micellar combined system was investigated for efficient and alkali/metal free base catalysis and to establish a simple method for separation and reuse of an organobase catalyst. Various aqueous organobase–surfactant micellar solutions were studied by using the Knoevenagel condensation of salicylaldehyde with active methylene compounds to 3-substituted coumarin as a model reaction. The 1-(2-pyrimidyl) piperazine (2-PP) was identified as a highly active organobase and its activity was further improved by using it in an aqueous solution of sodium dodecyl sulfate (SDS). The SDS micelles facilitate the reaction in water by solubilizing the 2-PP organobase and the reactants in their active forms. The reactant–SDS interactions in micelles play a significant role in promotion of the 2-PP catalyzed reaction. The SDS micellar medium not only promotes the 2-PP catalyzed reaction but also provides an easy and practicable protocol for the separation and reuse of the 2-PP organobase.

 Please wait while we load your content...
Please wait while we load your content...