Life cycle assessment of multi-step rufinamide synthesis – from isolated reactions in batch to continuous microreactor networks
Abstract
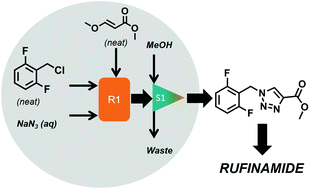
Rufinamide is an antiepileptic drug to treat Lennox–Gastaut syndrome, in combination with other medications. Rufinamide is one of the best-selling 5-membered ring heterocyclic pharmaceuticals. Its 1,2,3-triazole moiety is made by click chemistry-based cycloaddition of a dipolarophile and an azide. We have recently shown the feasibility of a continuous solvent- and catalyst-free flow process utilizing a relatively inexpensive and green new dipolarophile. The problem of its low reactivity was solved when harsh operating conditions (i.e. novel process windows, NPW) were applied within a continuous micro-flow reactor to obtain the activation needed. In addition to this chemical intensification, we present here the idea of using chlorides for azide formation instead of the commonly used (more reactive) bromides. Meanwhile, the chloride is produced by the reaction of benzyl alcohol and hydrogen chloride. Herein, we analyse the reaction sequence starting from benzyl alcohol to rufinamide, focusing on these three process optimisations. The choice of intermediates is assessed with the help of simplified green chemistry metrics and holistic life cycle assessment (LCA) about their impact on the full process chain. From those material-related green chemistry advancements, the next step in flow-based NPW is undertaken which is process-related (end-to-end process design). The reaction system is accordingly analysed with the goal being to determine the best fully continuous multi-reaction network, having uninterrupted flow from the first step until the end product is obtained. Three such multi-step microreactor networks are evaluated by green metrics and LCA, including telescoping in flow. Solvent recycling is considered throughout the investigations to reduce the solvent load.

 Please wait while we load your content...
Please wait while we load your content...