A step towards hydroformylation under sustainable conditions: platinum-catalysed enantioselective hydroformylation of styrene in gamma-valerolactone†
Abstract
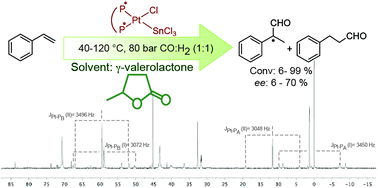
Platinum-catalysed enantioselective hydroformylation of styrene was performed in γ-valerolactone (GVL) as a proposed environmentally benign reaction medium. Optically active bidentate ligands, possessing various types of chirality elements e.g. central (BDPP), axial (BINAP, SEGPHOS, DM-SEGPHOS, DTBM-SEGPHOS) and planar/central (JOSIPHOS) elements, were applied in in situ generated Pt-diphosphine-tin(II)chloride catalyst systems. In general, slightly higher activities and regioselectivities towards a branched aldehyde (2-phenylpropanal) were obtained in toluene as a reference conventional solvent. However, higher chemoselectivities towards aldehydes (up to 98%) in GVL were obtained at lower temperatures. The application of GVL proved to be also advantageous regarding enantioselectivity: although moderate enantioselectivities were obtained in both solvents, in most cases higher ee values were detected in GVL. From the mechanistic point of view, the formation of different catalytic intermediates and/or different kinetics can be envisaged from the different temperature dependences of ee in GVL and toluene. The 31P-NMR characterization of catalyst species in GVL was also provided.

 Please wait while we load your content...
Please wait while we load your content...