Enantioselective aerobic oxidation of olefins by magnetite nanoparticles at room temperature: a chiral carboxylic acid strategy†
Abstract
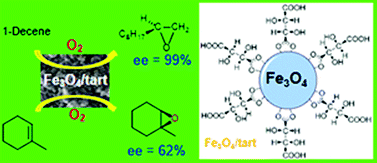
Asymmetric oxidations of organic compounds are limited in their synthetic scope and by practical factors, such as the use of complex catalyst synthesis. A simple and cheap nanostructured catalyst system comprising magnetite nanoparticles stabilized by L-(+)-tartaric acid (Fe3O4/tart-NPs) was successfully synthesized in diethylene glycol. The catalyst was characterized by FT-IR, TGA, ICP-AES, XRPD, SEM and dynamic light scattering (DLS). The catalytic activity of Fe3O4/tart-NPs dispersion in acetonitrile in the presence of isobutyraldehyde was studied in selective aerobic oxidation of olefins to form asymmetric epoxide, an important intermediate for the synthesis of biologically active compounds. In addition, the magnetically recoverable nanocatalyst Fe3O4/tart-NPs can be conveniently separated and recovered from the reaction system by applying an external magnetic field and reused for five cycles without the loss of activity after each cycle. These results demonstrate that the heterogeneous nanocatalysts possess potential applications for green and sustainable development. As synthesized nanoparticles of Fe3O4/tart-NPs are cheap and easy to synthesize asymmetric catalysts which were prepared without the involvement of difficult and cumbersome procedures for the synthesis of complicated asymmetric ligands. Possible reaction mechanisms were outlined.

 Please wait while we load your content...
Please wait while we load your content...