Microwave-assisted alcoholysis of furfural alcohol into alkyl levulinates catalyzed by metal salts†
Abstract
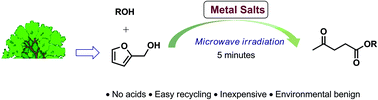
The production of alkyl levulinates from furfuryl alcohol (FAL) was investigated in the presence of metal salt catalysts under microwave irradiation. Various metal salts were tested in the reaction and Al2(SO4)3 showed excellent catalytic activity for the FAL alcoholysis, giving a 80.6% yield of methyl levulinate within 5 minutes. Various alcohols were used to obtain different alkyl levulinates. The dielectric properties of these alcohols were also measured to explain their different performances in the reaction. Microwave heating was proved to dramatically increase the reaction rate of the FAL alcoholysis compared to traditional oil heating. Identification of the reaction intermediates and products provided some insight into the reaction mechanism, where methoxymethylfuran (MMF) and 4,5,5-trimethoxypentan-2-one (TMP) were the key intermediates. Finally, the catalyst was recycled and reused 6 times without much drop in the activity.

 Please wait while we load your content...
Please wait while we load your content...