Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin†
Abstract
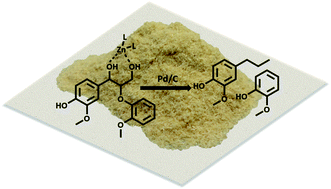
While current biorefinery processes use lignin only for its heat value, the conversion of lignin to high value chemicals is an area of increasing interest. Herein we present a detailed mechanistic study of the hydrodeoxygenation (HDO) of lignin by using a synergistic Pd/C and ZnII catalyst through use of both lignin model compounds and lignocellulosic biomass. Spectroscopic data coupled with the study of lignin model compounds suggest that ZnII activates and facilitates removal of the hydroxyl group at the Cγ position of the β-O-4 ether linkage. Activation is proposed to occur through formation of a six-membered ring complex of ZnII coordinated to the oxygen atoms at Cα and Cγ of the lignin model compound guaiacylglycerol-β-guaiacyl.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...