I−/TBHP catalyzed Csp3–N/Csp2–N bond formation via oxidative coupling with benzophenone imine in water†
Abstract
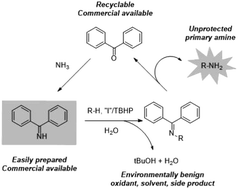
An I−/TBHP catalyzed oxidative amination with benzophenone imine under environmentally benign conditions was developed. A wide range of substrates underwent oxidative amination with benzophenone imine to afford the corresponding primary amine and amide products in good yields. The active iodine species were identified by well-designed control experiments for elucidating the mechanism.

 Please wait while we load your content...
Please wait while we load your content...