Broadening the chemical scope of laccases: selective deprotection of N-benzyl groups†
Abstract
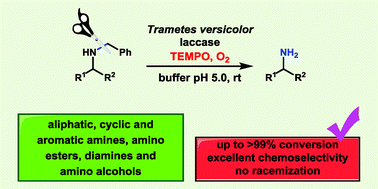
Laccase from Trametes versicolor together with TEMPO has been found to be a very efficient system to deprotect N-benzylated primary amines, differing from previously described methods since it uses oxygen as a mild oxidant in aqueous medium. Chemoselective removal of the benzyl group was achieved with excellent yields when secondary amines and alcohol moieties were also present.

 Please wait while we load your content...
Please wait while we load your content...